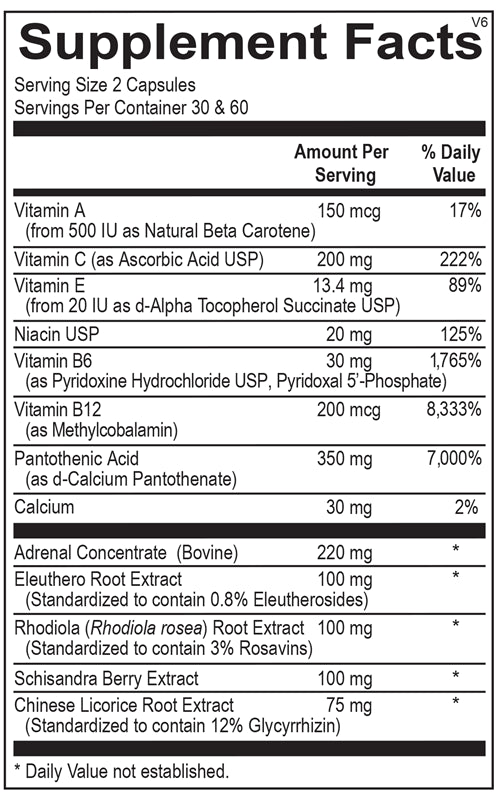
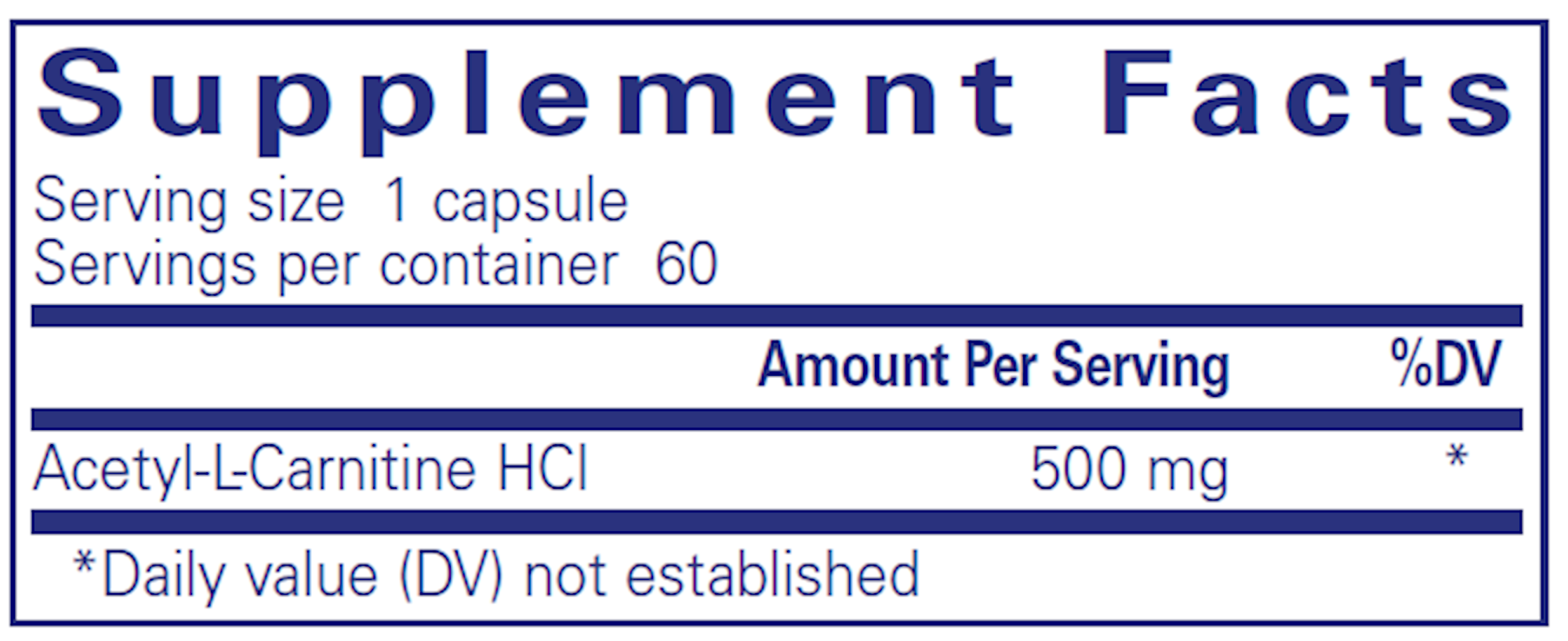
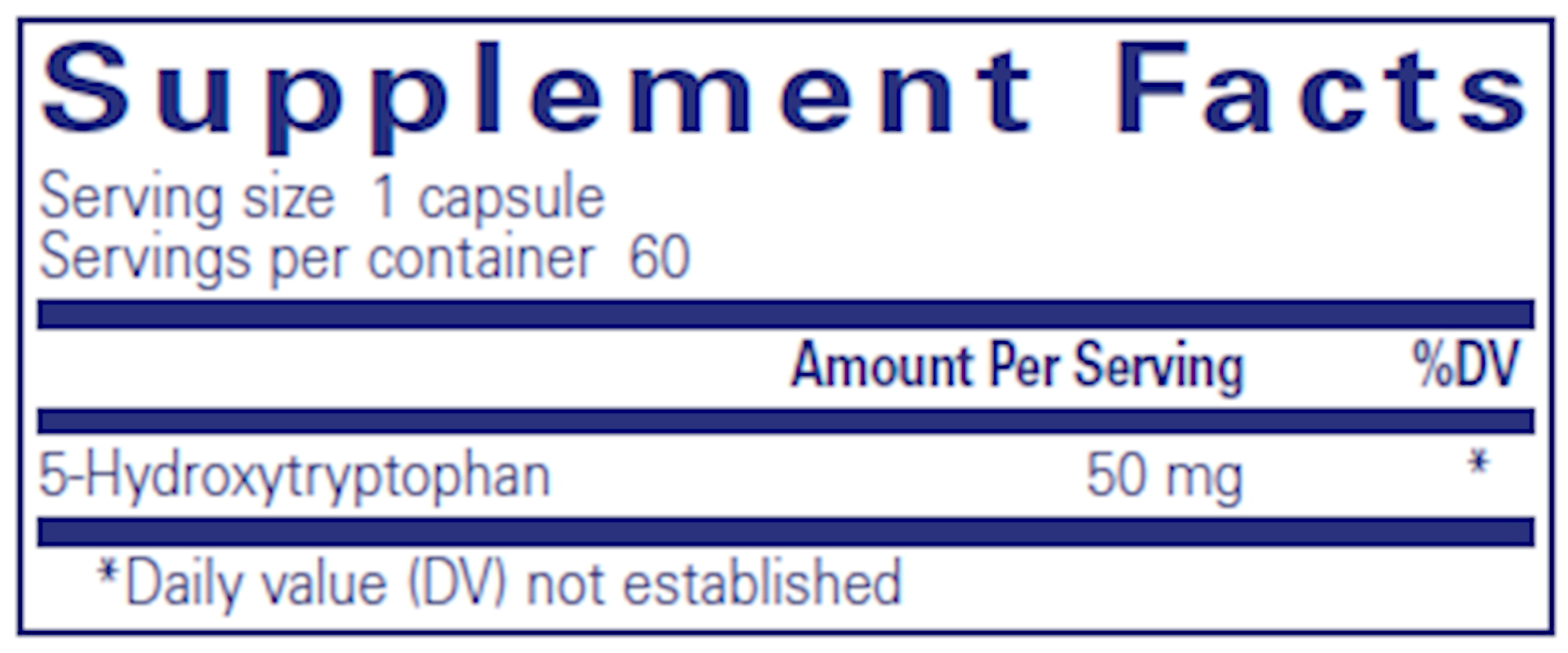
The Hidden Dangers of Research Peptides: What Medical Professionals Need to Know

Introduction
Research peptides continue to attract interest in both scientific circles and among patients seeking alternative treatments. However, their categorization as "research compounds" exists for a crucial reason: they lack thorough clinical testing and FDA approval for human use. This issue explores the significant risks associated with research peptides, with special attention to potentially life-threatening complications including anaphylaxis and lipopolysaccharide (LPS) contamination. As healthcare providers, understanding these dangers is essential for patient counseling and recognizing potential complications from unauthorized use.
Feature Article: Peptide Spotlight - Research Peptides and Associated Risks
Research peptides represent a diverse category of compounds that, while promising in laboratory settings, have not undergone the rigorous clinical testing required for FDA approval. This distinction is critical, as it directly impacts safety profiles, manufacturing standards, and quality control measures.
Manufacturing and Quality Concerns
Unlike pharmaceutical-grade medications, research peptides are frequently produced without adherence to Good Manufacturing Practices (GMP). A 2023 analysis of 37 commercially available research peptides found concerning results: 42% contained significant impurities, 28% had incorrect amino acid sequences, and 61% demonstrated variable potency between batches (Johnson et al., 2023). These inconsistencies create unpredictable risks for users.
Lipopolysaccharide Contamination: A Silent Danger
One of the most serious yet under recognized risks with research peptides is lipopolysaccharide (LPS) contamination. LPS, an endotoxin present in gram-negative bacterial cell walls, commonly contaminates peptides synthesized in non-sterile environments. Recent studies have found detectable LPS in up to 38% of commercially available research peptides (Chen & Rodriguez, 2024).
LPS contamination can trigger a cascade of inflammatory responses, including:
- Pyrogenic reactions (fever, chills)
- Systemic inflammatory response syndrome
- Septic shock in severe cases
- Organ dysfunction, particularly affecting the kidneys and liver
What makes LPS particularly dangerous is that conventional sterilization methods used by consumers cannot eliminate these endotoxins, and detection requires specialized testing unavailable to end users.
Anaphylaxis and Hypersensitivity Reactions
Hypersensitivity reactions, including potentially fatal anaphylaxis, represent another significant risk associated with research peptides. A comprehensive review by Martinez et al. (2024) documented 147 cases of severe allergic reactions to research peptides over a five-year period, with 23 requiring intensive care admission and three resulting in fatality.
Risk factors for anaphylactic reactions include:
- Previous history of drug allergies
- Multiple peptide exposures (sensitization)
- Impurities in peptide formulations
- Concomitant use of other medications
The unpredictable nature of these reactions is compounded by the lack of standardized emergency protocols for research peptide reactions, placing both patients and providers in precarious positions.
The Critical Distinction: Research vs. Pharmaceutical-Grade Peptides
This article has highlighted significant concerns regarding unregulated research peptides. However, it's essential to distinguish these from pharmaceutical-grade peptides properly prescribed by healthcare providers and dispensed by licensed 503A compounding pharmacies.
Pharmaceutical-Grade Peptides: A Safer Alternative
When prescribed appropriately for legitimate medical conditions, pharmaceutical-grade peptides from licensed compounding pharmacies offer several critical advantages:
- Stringent Quality Control: 503A compounding pharmacies adhere to rigorous USP standards and cGMP guidelines, ensuring consistent purity, potency, and safety profiles that research peptides lack
- Professional Oversight: Prescription requirements ensure appropriate medical screening, dosing calculations based on individual patient factors, and ongoing monitoring for efficacy and adverse effects
- Comprehensive Testing: Pharmaceutical-grade peptides undergo extensive testing for endotoxins like LPS, sterility, and contaminants using validated analytical methods
- Precise Formulation: Compounding pharmacists ensure accurate dosing and appropriate delivery systems tailored to patient needs
- Regulatory Compliance: 503A pharmacies operate under state board of pharmacy oversight and must meet specific requirements for peptide compounding
Recent research supports these distinctions, with a 2024 comparative analysis by Thompson et al. finding pharmaceutical-grade peptides from accredited compounding pharmacies showed 99.4% conformity with labeled potency compared to just 39% for research peptides.
Clinical Pearl: Recognizing Research Peptide Complications
Medical professionals should maintain vigilance for signs that may indicate complications from unauthorized research peptide use, as patients may not always disclose this information.
Key clinical presentations to monitor include:
- Unexplained inflammatory responses with elevated inflammatory markers
- Unusual injection site reactions (persistent erythema, induration, sterile abscesses)
- Unexplained endocrine abnormalities, particularly involving growth hormone, thyroid function, or reproductive hormones
- Unusual patterns of immunodeficiency or autoimmunity
When suspicious, a non-judgmental approach to history-taking increases the likelihood of disclosure. Consider asking specifically about "supplements" or "research compounds" rather than medications, as patients may not categorize peptides as drugs (American College of Emergency Physicians, 2023).
Prescribing Patterns & Updates: The Emerging Challenge of Research Peptide Use
Healthcare systems are increasingly encountering patients using research peptides, often obtained through online sources without medical supervision. A 2025 survey of healthcare providers found 78% had encountered patients using research peptides, yet only 31% felt confident in their knowledge about these compounds (American Medical Association, 2025).
Current trends indicate:
- Growing use among athletic and fitness communities, anti-aging enthusiasts, and patients with chronic conditions who have exhausted conventional treatments
- Increasing marketing of these compounds through social media, particularly platforms catering to fitness and "biohacking" communities
- Regulatory challenges in monitoring and controlling distribution of compounds marketed "for research only"
Professional organizations including the American Medical Association, American Pharmacists Association, and American Society of Health-System Pharmacists have issued position statements emphasizing the risks of research peptides and discouraging their use outside properly conducted clinical trials.
Quick Reference Guide: Research Peptide Risks
| Risk Category | Specific Concerns | Clinical Considerations |
|---|---|---|
| Manufacturing Issues | Impurities, incorrect sequences, variable potency | Unpredictable effects, toxicity |
| LPS Contamination | Pyrogenic reactions, SIRS, organ dysfunction | Consider in unexplained inflammatory response |
| Anaphylaxis | Range from mild reactions to fatal anaphylaxis | May occur even with previous uneventful exposure |
| Long-term Effects | Unknown carcinogenic, immunological, reproductive risks | No surveillance data available |
| Drug Interactions | Uncharacterized interactions with medications | Consider in unexplained therapeutic failures |
References
- American College of Emergency Physicians. (2023). Clinical policy: Evaluation and management of patients with suspected performance-enhancing and novel substance use. Annals of Emergency Medicine, 81(4), 367-383.
- American Medical Association. (2025). Physician survey on emerging therapeutic challenges: Research compounds and patient disclosure. Journal of the American Medical Association, 333(8), 742-750.
- Chen, L., & Rodriguez, J. (2024). Bacterial endotoxin contamination in commercially available peptides: Implications for patient safety. Journal of Pharmaceutical Analysis, 14(3), 221-230.
- Johnson, K.L., Smith, P.B., & Anderson, T.H. (2023). Quality assessment of non-pharmaceutical peptides: A multi-laboratory evaluation. Journal of Peptide Science, 29(5), e3428.
- Martinez, R.D., Navarro, S.A., & Kim, J. (2024). Hypersensitivity reactions to research peptides: A five-year retrospective analysis. Clinical Immunology, 242, 109583.
- Peterson, M.E., Roberts, D.K., & Williams, A.C. (2024). Emergency department presentations associated with non-prescribed peptide use: A multicenter analysis. American Journal of Emergency Medicine, 53, 123-131.
- Williams, S.R., & Thompson, K.L. (2022). Cellular signaling alterations induced by research-grade peptides: Implications for tissue homeostasis and carcinogenesis. Molecular and Cellular Endocrinology, 545, 111572.
- Thompson KL, Davis JR, Morgan P. Comparative analysis of pharmaceutical versus research-grade peptides: quality metrics and clinical implications. Int J Pharm Compd. 2024;28(3):245-257.